Genetic testing for breast cancer (BC)
What is genetic testing for breast cancer?
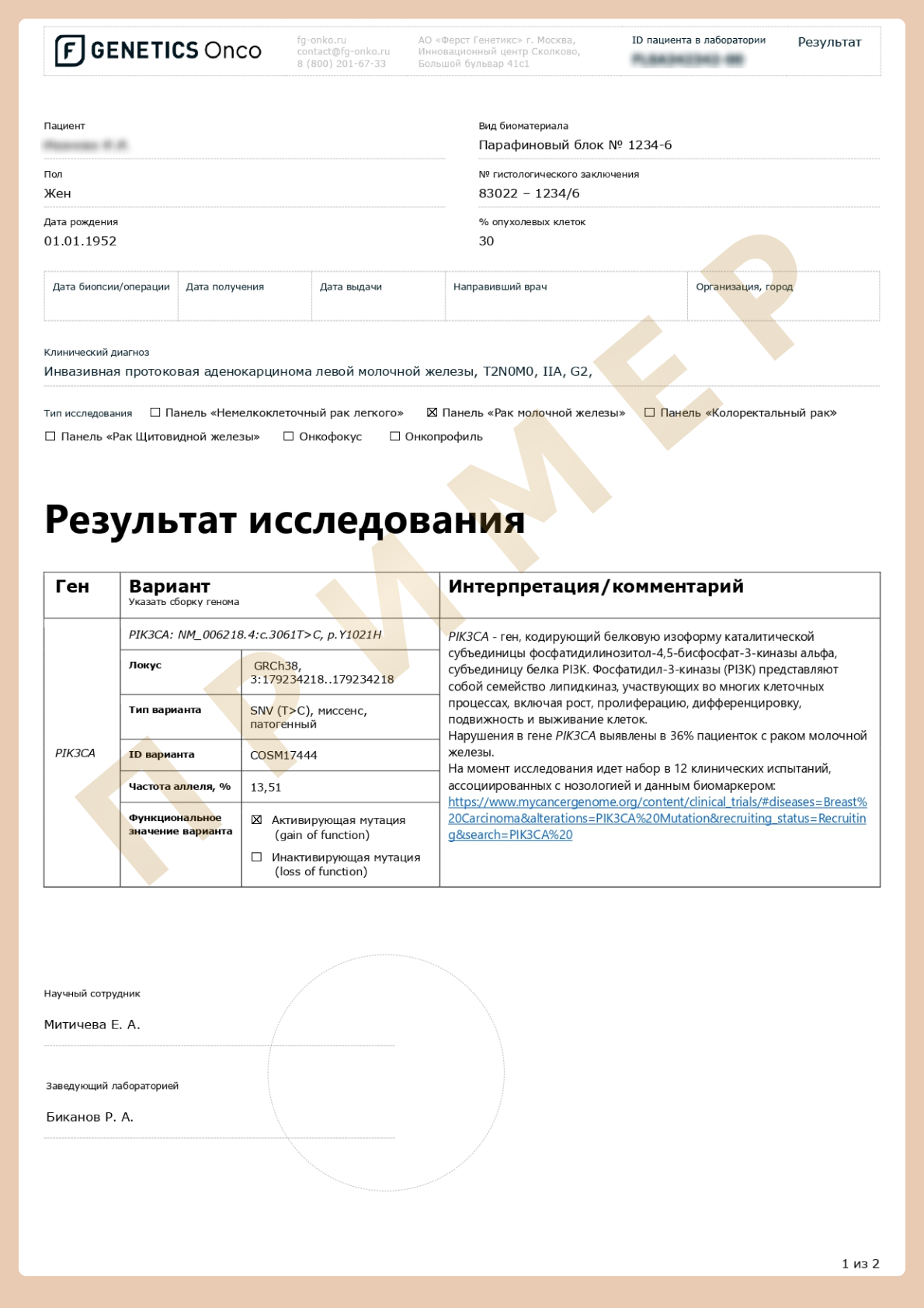
An NGS-based assay includes a panel of genes concurred with the clinical use of approved targeted drugs and drugs from prospective clinical trials for breast cancer.
Alterations in these genes lead to tumor development and are used as diagnostic, prognostic, and predictive cancer markers.
"First Genetics Lab" performs testing using the high‑throughput sequencing system named "F‑Genetics" and reagents for high‑throughput next generation sequencing (NGS) of genes associated with human cancer genesis, that are registered as medical devices (Federal Service for Surveillance in Healthcare: РЗН 2020/10521, РЗН 2022/16970)
The assay detects:
- Hotspots (SNV/InDels)
- Copy number variations (CNV)
- Chromosomal aberrations (Gene fusions)
Indications for testing
For patients diagnosed with breast cancer for:
- Therapy selection
- Therapy monitoring and correction if the cancer recurrence occurs
- Clinical trials enrollment
Targeted therapy
Targeted therapy is the type of treatment that targets specific molecules/proteins on the cancer cell surface or inside the cancer cells, blocking the growth and spread of cancer cells and limiting damage to healthy cells.
Not all tumors have the same targets. Genetic tests are used to find out what treatment is the most effective. Many clinical studies are underway.
Targeted therapy. Indications for drugs
TARGETED THERAPIES FOR RECURRENT UNRESECTABLE OR STAGE IV (M1) breast cancer
(NCCN Guidelines Version 3.2022)
| Breast Cancer Subtype | Biomarker | FDA-Approved Agents |
|---|---|---|
| Любой | HER2 |
Trastuzumab
Pertuzumab
Margetuximab
Ado-trastuzumab emtansine
Fam-trastuzumab deruxtecan-nxki
|
| Any | BRCA1/BRCA2 mutation |
Olaparib
Talazoparib
|
| HR-positive/HER2-negative | PIK3CA activating mutation |
Alpelisib + fulvestrant
|
| TNBC | PD-L1 expression |
Pembrolizumab + chemotherapy (albumin-bound paclitaxel, paclitaxel, or gemcitabine and carboplatin)
|
| Any | NTRK fusion |
Larotrectinib
Entrectinib
|
| Any | MSI-H/dMMR* |
Pembrolizumab
Dostarlimab-gxly
|
| Any | TMB-H (≥10 mut/Mb)** |
Pembrolizumab
|
* microsatellite instability / deficiency of the DNA mismatch repair system
** high tumor mutation burden
NGS method
NGS can simultaneously read hundreds of millions of short sequences of DNA and detect all types of mutations, including

Hotspots (SNV/InDels)

Copy number variations (CNV)

Chromosomal aberrations (Gene fusions)
Advantages
- High sensitivity and specificity of the method
- Fast turnaround time
- Simultaneous detection of all types of mutations, including chromosomal aberrations
- Minimum requirements for sample quantity and quality
- Shorter testing times
How to request the genetic testing for breast cancer
Download and fill out the informed consent form
A courier will collect histological material and take it to the laboratory free of charge.
First Genetics Laboratory
Specialists
Years of experience in genetics, laboratory diagnostics and bioinformatics
Confidentiality
All data is strictly confidential and cannot be passed on to third parties
Time frame
Results ready in a short time
Security
Extensive control at each stage of testing
No delivery fees
Free delivery of biomaterial across Russia
Charities
Email info@f-genetics.com for information