Sanger sequencing
The DNA molecule is the carrier of genetic information in almost all living organisms. It is made up of two complementary, i.e. corresponding strands of nucleotides that form a unique sequence.
A pair of complementary nucleotides within a DNA sequence is also called a base pair, abbreviated as bp.
The human genome contains about 3 billion base pairs.
Sanger sequencing
Sanger sequencing is one of the classic sequencing methods. The method was developed in 1977 by Frederick Sanger and his colleagues. It was used by the Human Genome Project in 1990-2003 to fully decode the human genome for the first time.
Sanger sequencing is still relevant today and is a routine method both in genetic diagnosis and in research practice. It can be used to ’read’ sequences of up to 1000 base pairs in a single cycle with a high accuracy of up to 99%.
The Sanger sequencing method is now fully automated. Automation makes it reliable and easy to perform, and it is the ’gold standard’ of modern sequencing. With automation and increased sequencing capacity, the cost of the method has also decreased.
Medical indications
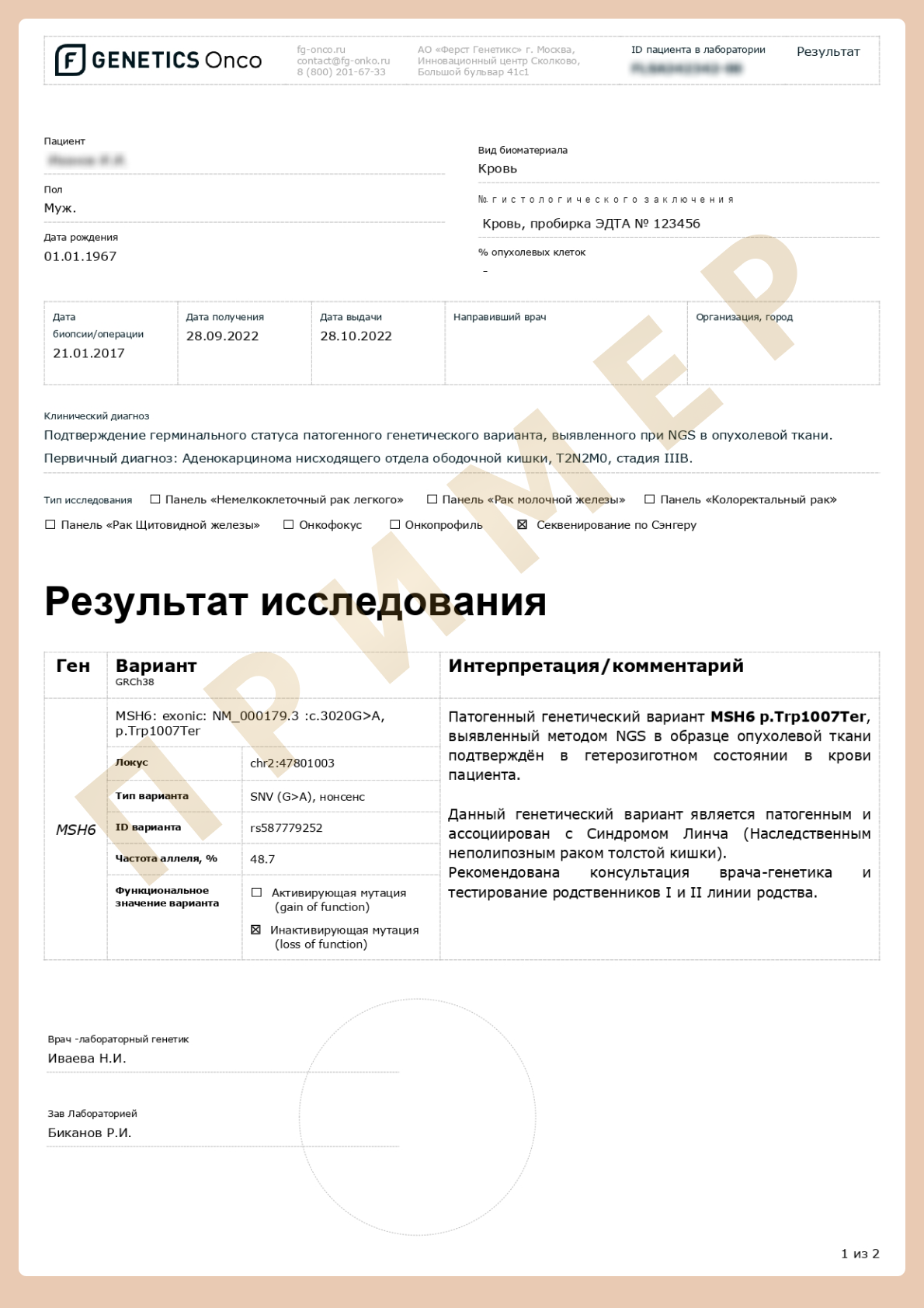
- To confirm NGS data when pathogenic variants or variants of unknown clinical significance are detected.
- To establish a patient’s genetic status when a relative has already been identified with a mutation and its exact position in the genome is known.
Features of the Sanger sequencing method
The Sanger sequencing method is based on the use of dideoxynucleotides, also known as «terminator» nucleotides.
1. Material preparation
In a test tube, a DNA synthesis reaction is carried out using an already existing matrix (the single-stranded genetic sequence of a particular individual), the sequence of which needs to be sequenced.
2. Polymerase chain reaction
The DNA molecule is synthesised by the DNA polymerase enzyme, which, according to the matrix, inserts nucleotides one by one into the growing chain.
Among all the nucleotides, there is 1% of «terminators», each of which also carries a special fluorescent tag.
Once such nucleotides are incorporated, further chain synthesis becomes impossible. Since the insertion of a labelled «terminator» nucleotide into a growing DNA strand is a random event, it is equally likely to occur either at the beginning or after most of the complementary molecule has been synthesised.
As a result, a mixture of unique DNA fragments of different lengths is formed, which begin the same way, but end at different positions along the DNA strand matrix. And they end at one of four nucleotide tags.
3. Fragment separation and signal detection
These fragments are then separated by size using capillary electrophoresis.
The synthesised DNA fragments are separated by the electric field: the smaller the fragment, the faster it moves in the gel.
This way the fragments «line up» along the length in the capillary.
A fluorescent nucleotide at the end of each fragment is detected and the complete nucleotide sequence of the DNA sample examined is determined.
NGS vs Sanger
Since the invention of Sanger sequencing, the search and development of alternative methods of DNA sequencing has continued. Recently, Next Generation Sequencing (NGS) methods have become very popular. However, each technology has its own characteristics and the choice of method is usually based on the task at hand.
The Sanger sequencing method has its limitations
- It can only be used to read DNA sequences of up to 1000 base pairs from one person at a time.
- In addition, it is not possible to detect changes in a certain cell population when most of the sample represents a normal copy of the gene. For example, changes occur in tumour cells that cause them to grow uncontrollably. Such changes will not be present in any other cells in the body.
- Sanger sequencing allows changes to be detected only if they appear in at least 15-20% of the DNA tested.
Next generation sequencing methods
Allow to obtain data on longer DNA sequences (up to whole genome sequencing) and detect variants (mutations) contained in about 5% of the studied DNA. However, this is done by simultaneously reading a large number of short (100-300 bp on average for the methods most commonly used in clinical laboratories) DNA sequences, which are then combined and analysed.
- The processing of such data is labour-intensive and requires a high level of expertise.
- So, the accuracy of interpretation of individual sections depends on how many times that section has been ’read’.
- In cases where the identified variant is suspected of being the cause of a disease, 100% certainty of a correct reading is required. Then there is confirmation of the presence of a pathogenic or opportunistic variant by the Sanger method.
- NGS methods are also expensive, making it not cost-effective to analyse short sequences.
- Therefore, when the exact coordinates of a suspected change in the genome are known (e.g. if a pathogenic or opportunistic variant has been detected in a close relative), whole exome sequencing is not necessary. You can simply sequence the DNA section of interest using the Sanger method.
- 5% of the tested material must contain changes so that they can be detected by the New generation method (NGS).
How to order Sanger sequencing?
Download and fill out the consent form
The blood sample should be taken to a EDTA blood collecting tube (purple cap) in any laboratory in your city. Please ensure your name and date appropriately label the collection tube with blood!
The courier will pick up the blood sample and take it to the laboratory for free.
First Genetics Laboratory
Specialists
Years of experience in genetics, laboratory diagnostics and bioinformatics
Confidentiality
All data is strictly confidential and cannot be passed on to third parties
Time frame
Results ready in a short time
Security
Extensive control at each stage of testing
No delivery fees
Free delivery of biomaterial across Russia
Charities
Email info@f-genetics.com for information