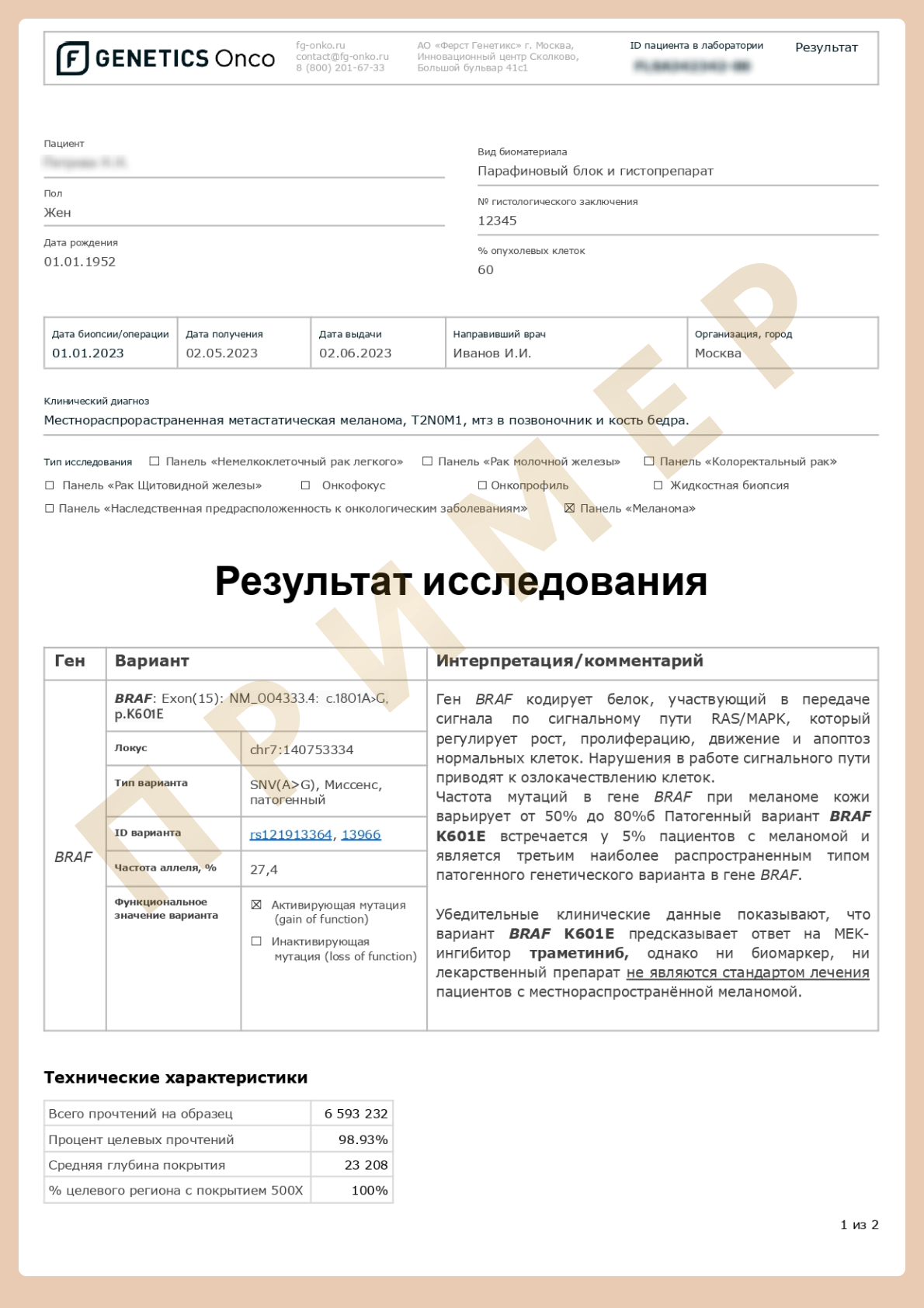
Genetic testing for cutaneous melanoma
Melanoma is a separate type of skin cancer that starts in pigment cells called melanocytes.
Although it is less common (less than 5% of all skin cancers) than basal cell carcinoma and squamous cell carcinoma, this skin cancer is more dangerous due to its ability to metastasize rapidly (80% of all skin cancers are lethal).
Distribution of cases by stage
(data for 2017):
Both intermittent and chronic exposure to the sun (UV) increases the rate of somatic mutation and leads to de novo tumor development, and promotes the transformation of pre-existing nevi into melanoma. UV increases the mutation load in cells, as well as locally and systemically suppresses the immune system.
A family history of this disease greatly increases the risk of its development. Pathogenic variants in the CDKN2A and CDK4 genes related to the cell cycle disorder in melanocytes are associated with the development of a hereditary form of melanoma.
Cutaneous
Non-cutaneous
Mutations in BRAF, in particular BRAFV600E, are a typical predictor of response to RAF inhibitors. However, disease progresses almost invariably in these cases at different intervals, and some patients may show primary resistance to BRAF inhibitors (+/− MEK). Studies have described the important role of acquired genetic changes affecting the Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways in inducing resistance to both chemotherapy and targeted therapies in melanoma.
In particular, the mechanisms responsible for resistance to BRAF inhibitors (+/− MEK) can be divided into:
Genomic
- NRAS/KRAS mutation — 20%,
- BRAF splicing variants — 16%,
- BRAF amplification — 13%,
- MEK1/2 mutation — 7 %,
- bypass mutations — 11%.
Immunological
- epigenetic and transcriptome changes from molecules involved in antigen presentation mechanisms.
Combinations of both
What is melanoma genetic testing?
This is an NGS-based analysis of tumor material of patients with locally advanced or metastatic melanoma that includes genes relevant for prescribing approved targeted drugs as well as drugs from prospective clinical trials.
Abnormalities in these genes causes tumor development and are used as diagnostic, prognostic, and predictive markers of melanoma.
The analysis detects:
- Hotspots (SNV/indels)
- Copy number variations (CNV)
- Chromosomal aberrations (Gene fusions)
Indications for testing
patients diagnosed with melanoma:
- Therapy selection
- Therapy monitoring and correction in case of therapy resistance
- Clinical trials enrollment
Molecular technologies for the diagnosis and prognosis of skin melanoma
(NCCN Guidelines Version 2.2023)
Melanocyte neoplasms of uncertain biological potential present a unique challenge for pathologists and clinicians. Ancillary tests for differentiating benign from malignant melanocytic neoplasms include immunohistochemistry (IHC) and molecular testing, using in particular Next-Generation Sequencing (NGS). These tests may contribute more accurate diagnosis and optimization of therapy in cases that are diagnostically uncertain or histopathologically inconsistent. Ancillary tests should be used as an adjunct to clinical and expert dermatopathological examination and therefore interpreted in the context of these findings.
Testing for somatic mutations
In case of skin melanoma, a number of somatic genetic alterations have been identified that are useful in making treatment decisions and/or eligibility for inclusion in clinical trials.
Specific mutations (BRAF, NRAS, KIT)
BRAF mutations
(B-Raf proto-oncogene)
BRAF is a serine/threonine kinase that activates the mitogen-activated kinase pathway. Mutations in this gene result in uncontrolled cell growth and proliferation. Some clinical features are associated with a higher frequency of BRAF mutations (eg, occasional sun exposure, younger age, tumor location on the body), but they should not be used either as an indirect confirmation of these mutations, or to make a decision about testing.
BRAF mutations are most commonly found at codon 600 (V600), most commonly in V600E (80%), but also include V600K (15%) and V600R/M/D/G (5%).
BRAF V600 mutations are associated with susceptibility to BRAF inhibitors. Available data suggest that BRAF inhibitors should not be used in patients without activating mutations in BRAF. Mutations in BRAF V600 are also associated with susceptibility to MEK inhibitors. Clinical trials have shown that the combination of BRAF and MEK inhibitors is superior to either agent alone in patients with BRAF V600.
Extensive data from clinical studies have shown that, compared with BRAF V600E, patients with metastatic melanoma mutated in BRAF V600K may have slightly less response/benefit when treated with BRAF ± MEK inhibitors. Less frequent mutations affecting codon 600 (including V600R/ M/D/G) may also benefit from these treatments.
BRAF mutations beyond codon 600 (BRAF mutations other than V600) and BRAF fusions (chimeric genes) are also found in about 5%.
Mutations in codons near V600 in exon 15 (in particular, BRAF L597 and BRAF K601) have demonstrated a response to MEK and BRAF inhibitors and combinations of MEK inhibitors.
Fusions in BRAF have also shown a response to MEK inhibitors and non-specific RAF inhibitors (eg, sorafenib).
Mutations in other codons of exon 11 or exon 15 have not responded to either BRAF inhibitors or MEK.
Mutations of KIT (proto-oncogene c-KIT)
KIT is a receptor tyrosine kinase that promotes cell growth and proliferation. KIT mutations are present in 10-15% of mucosal and acral melanomas (i.e., hairless surfaces of the palms and soles, nail pads). They are also present in 2 −3% of cases on skin with continuous UV exposure, but very rarely on skin with intermittent sun exposure Thus, clinical features may detrmine the decision to test for KIT mutations.
KIT mutations can occur at multiple ’hot spots’ across the gene and vary in their susceptibility to KIT inhibitor therapy (eg, imatinib, sunitinib, nilotinib).
Mutations in exons 11 and 13 of KIT (eg, W557R, V559D, L576P, K642E) seem to have a high level of sensitivity to KIT inhibition.
KIT exon 17 mutations (eg, D816H) appear to have minimal or no sensitivity to KIT inhibitors.
KIT amplifications seem to have minimal or no sensitivity to KIT inhibitors.
Under certain circumstances:
Cabozantinib — if progressed after taking lenvatinib and/or sorafenib.
Larotrectinib or entrectinib — for patients with advanced solid tumors with a positive NTRK gene fusion.
Selpercatinib or pralsetinib — for patients with RET-positive tumors.
Pembrolizumab — for patients with high mutation load (TMB-H) ((≥10 [mut/Mb]).
Clinical Trials.
Other therapies may be considered for progressive and/or symptomatic disease if clinical trials or other systemic therapies are not available or appropriate (eg, axitinib, everolimus, pazopanib, sunitinib, vandetanib, vemurafenib [BRAF+], or dabrafenib [BRAF+]).
NRAS mutations
(NRAS proto-oncogene)
NRAS is a GTPase that activates mitogen-activated protein kinase signaling as well as other signaling pathways causing cell growth and proliferation.
NRAS mutations correlate with poor survival in localized and advanced melanoma.
NRAS mutations are present in about 15% of melanomas of the skin with chronic and intermittent sun exposure, acral melanoma, and mucosal melanoma.
MEK inhibitors may respond in a minority of patients with NRAS mutations.
The likelihood of overlapping targeted mutations (NRAS, BRAF and KIT) is low.
Other genetic alterations detected by NGS
Fusions in NTRK1, NTRK2, and NTRK3 are rare (<1%) in different subtypes of melanoma.
Fusions of ALK and ROS1 are rare (<1% of cases) in different subtypes of melanoma.
Case study or limited clinical trial data indicate the proposed efficacy of therapy (larotrectinib or entrectinib for NTRK fusion, crizotinib or entrectinib for ROS1 fusion, or trametinib for BRAF fusion and crizotinib for ALK fusion).
Indications for genetic testing
BRAF or NGS testing is not recommended for resected stage I-II skin melanoma unless it serves as the basis for participation in clinical trials.
BRAF mutation testing is recommended in high-risk stage III patients for whom BRAF-targeting therapy may be recommended in the future.
Broader genomic profiling is recommended where possible (eg, extended NGS panels including non-V600 BRAF mutations), especially if test results may influence future treatment decisions or clinical trial eligibility.
If the initial study was testing for a single BRAF gene and the result is negative, clinicians should strongly consider extended NGS panels to identify other potential genetic targets (eg, KIT, BRAF non-V600).
NGS method
NGS can simultaneously read hundreds of millions of short sequences of DNA and detect all types of mutations, including:

Hotspots (SNV/indels)

Copy number variations (CNV)

Chromosomal aberrations (gene fusions)
Advantages
- High sensitivity and specificity of the method
- Simultaneous detection of all types of mutations, including chromosomal aberrations
- Minimum requirements for sample quantity and quality
- Fast turnaround time
How to request the genetic testing for Melanoma?
Download and fill out the informed consent form
A courier will collect histological material and take it to the laboratory free of charge.
First Genetics Laboratory
Specialists
Years of experience in genetics, laboratory diagnostics and bioinformatics
Confidentiality
All data is strictly confidential and cannot be passed on to third parties
Time frame
Results ready in a short time
Security
Extensive control at each stage of testing
No delivery fees
Free delivery of biomaterial across Russia
Charities
Email info@f-genetics.com for information